The government’s new order made on Monday allowing the orders, is yet to be notified publicly, however.
The government on Monday decided to revoke a 2-day old blanket ban on exporting the drug hydroxychloroquine, sources said. The decision came after both U.S. President Donald Trump and Brazilian President Jair Bolsonaro announced that they had requested India to allow the supply of the drug hydroxychloroquine (HCQ), now being used as a possible treatment for COVID-19.
According to the sources, the government had lifted the ban in order to clear existing orders, “depending on availability of stock after meeting domestic requirements”, and that the Department of Pharmaceuticals and Ministry of External Affairs will decide on allocations to countries depending on the “humanitarian-COVID situation”. The government’s new order made on Monday allowing the orders, is yet to be notified publicly, however.
According to officials, more than two dozen countries have requested supplies of HCQ at the “highest level” in the past few days, but the US and Brazil had stressed that they have made advance payments on their orders.
“I asked the PM of India for his support in continuing the supply of pharmaceutical inputs for the production of hydroxychloroquine. We will spare no effort to save lives,” Mr. Bolsonaro tweeted.
Earlier, Mr. Trump said he had called Mr. Modi to release the “hold” put on the export of the anti-malarial drug, which is being seen as a possible prophylactic for the novel coronavirus infection, and that New Delhi was giving his request “serious consideration”.
Industry groups had also appealed to the government to reconsider the ban.
“We have given an assurance that the domestic consumption will be looked after first, but after that we should consider the needs of other countries too. After all, India’s reputation as the pharmacy of the world is built on our ability to manufacture these medicines that are much needed. We must think of that as well as the commitments we have given other countries,” Ashok Madan, the Executive Director of the Indian Drug Manufacturers Association (IDMA), told The Hindu. He added that the industry has enough stocks for both domestic needs and international requirements as of now.
However, advocacy groups are warning that the government must not overlook the possibility of an “escalation” in domestic demand, and point out that the Indian Council of Medical Research (ICMR) has added HCQ to its protocol for all health workers. “If they remove restrictions on export now, we could face a shortage internally…Even if it isn’t a certified cure, it is being used as a prophylaxis by our doctors; we must think of them,” said Malini Aisola of the All India Drug Action Network.
Industry sources said that last month, the U.S., Brazil, neighbouring SAARC and European Union countries had placed advance orders for the drug, which is made by only a few Indian companies, most notably IPCA and Zydus Cadila.
In various investor calls, the companies had said they were ramping up production of the pharmaceutical as much as five times after the U.S. Food and Drug Administration authorised the use of HCQ in treatment trials in combination with azithromycin, as well as a prophylactic for health workers dealing with COVID-19 patients.
On March 25, as word about the drug spread, the DGFT banned exports, allowing only pre-existing orders, batches manufactured inside SEZ areas, and those considered for “humanitarian needs” by the Ministry of External Affairs for export. On April 4, however, it cancelled all the exemptions, leaving suppliers who had prepared the orders, as well as countries that had made advance payments for the drug in the lurch.
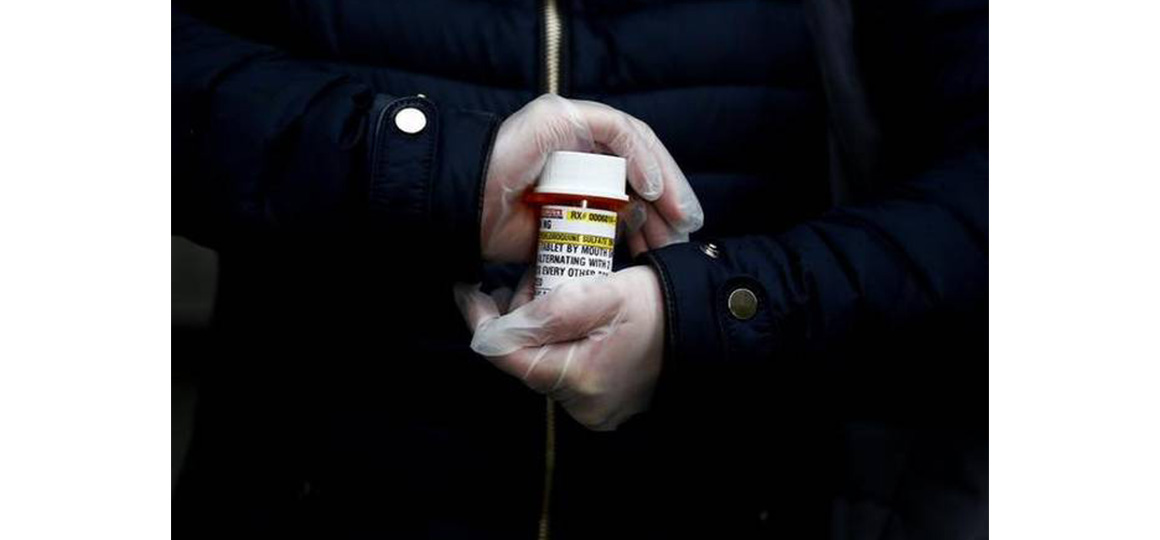
In addition to its possible use for in the current COVID-19 pandemic, HCQ is anti-malarial drug, which is also used by patients of lupus and rheumatoid arthritis in India.
Another worry is that the essential ingredients for HCQ come from China, and any disruption in supply or increase in cost of those will also reduce India’s manufacturing capacity of the drug.
Coronavirus | Govt gives in to demands from US, Brazil, revokes blanket ban on hydroxychloroquine exports
The government’s new order made on Monday allowing the orders, is yet to be notified publicly, however.
The government on Monday decided to revoke a 2-day old blanket ban on exporting the drug hydroxychloroquine, sources said. The decision came after both U.S. President Donald Trump and Brazilian President Jair Bolsonaro announced that they had requested India to allow the supply of the drug hydroxychloroquine (HCQ), now being used as a possible treatment for COVID-19.
According to the sources, the government had lifted the ban in order to clear existing orders, “depending on availability of stock after meeting domestic requirements”, and that the Department of Pharmaceuticals and Ministry of External Affairs will decide on allocations to countries depending on the “humanitarian-COVID situation”. The government’s new order made on Monday allowing the orders, is yet to be notified publicly, however.
According to officials, more than two dozen countries have requested supplies of HCQ at the “highest level” in the past few days, but the US and Brazil had stressed that they have made advance payments on their orders.
“I asked the PM of India for his support in continuing the supply of pharmaceutical inputs for the production of hydroxychloroquine. We will spare no effort to save lives,” Mr. Bolsonaro tweeted.
Earlier, Mr. Trump said he had called Mr. Modi to release the “hold” put on the export of the anti-malarial drug, which is being seen as a possible prophylactic for the novel coronavirus infection, and that New Delhi was giving his request “serious consideration”.
Industry groups had also appealed to the government to reconsider the ban.
“We have given an assurance that the domestic consumption will be looked after first, but after that we should consider the needs of other countries too. After all, India’s reputation as the pharmacy of the world is built on our ability to manufacture these medicines that are much needed. We must think of that as well as the commitments we have given other countries,” Ashok Madan, the Executive Director of the Indian Drug Manufacturers Association (IDMA), told The Hindu. He added that the industry has enough stocks for both domestic needs and international requirements as of now.
However, advocacy groups are warning that the government must not overlook the possibility of an “escalation” in domestic demand, and point out that the Indian Council of Medical Research (ICMR) has added HCQ to its protocol for all health workers. “If they remove restrictions on export now, we could face a shortage internally…Even if it isn’t a certified cure, it is being used as a prophylaxis by our doctors; we must think of them,” said Malini Aisola of the All India Drug Action Network.
Industry sources said that last month, the U.S., Brazil, neighbouring SAARC and European Union countries had placed advance orders for the drug, which is made by only a few Indian companies, most notably IPCA and Zydus Cadila.
In various investor calls, the companies had said they were ramping up production of the pharmaceutical as much as five times after the U.S. Food and Drug Administration authorised the use of HCQ in treatment trials in combination with azithromycin, as well as a prophylactic for health workers dealing with COVID-19 patients.
On March 25, as word about the drug spread, the DGFT banned exports, allowing only pre-existing orders, batches manufactured inside SEZ areas, and those considered for “humanitarian needs” by the Ministry of External Affairs for export. On April 4, however, it cancelled all the exemptions, leaving suppliers who had prepared the orders, as well as countries that had made advance payments for the drug in the lurch.
In addition to its possible use for in the current COVID-19 pandemic, HCQ is anti-malarial drug, which is also used by patients of lupus and rheumatoid arthritis in India.
Another worry is that the essential ingredients for HCQ come from China, and any disruption in supply or increase in cost of those will also reduce India’s manufacturing capacity of the drug.




NO COMMENT